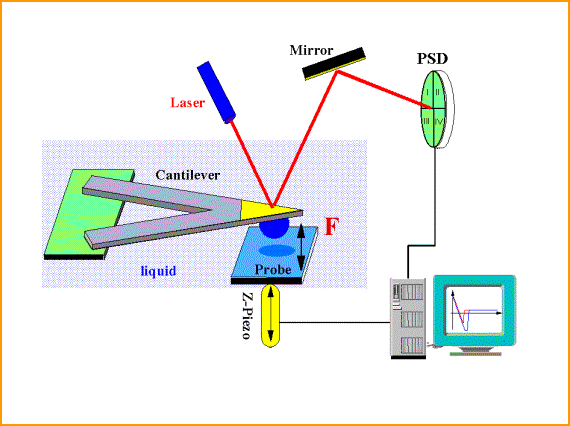
To enhance the ability to manipulate objects at the nanoscale, through self-assembly techniques and the development of soft nanotechnological tools, its is required to thoroughly elucidate the forces that guide the assembly on the nanoscale. The core of the Sigmund Group’s research is directed towards improved measurements and understanding of surface forces such as van der Waals, electric double layer, and polymer-induced forces. Investigation of these forces is critical to future developments in nanotechnology; the interactions between surfaces control a range of phenomena including: dispersion, agglomeration, adhesion, coating, and polishing. The colloid probe technique in an atomic force microscope is a powerful method for the characterization of interfacial phenomena by measuring forces and distance between two surfaces.

Special focus: repulsive van der Waals forces
Van der Waals forces are always present between atoms or between molecules, and are always attractive for like materials. Attractive van der Waals forces have been used to explain why neutral chemically saturated atoms congregate to form liquids and solids. Attractive van der Waals forces are difficult to quantitatively measure with any surface force technique since the forces increase by orders of magnitude close to the surface. This causes a jump in of the measuring probe preventing data collection. Hamaker1 predicted repulsive van der Waals forces in his paper from 1937. Repulsive van der Waals forces are expected to be observable in certain dislike materials combinations. Using an atomic force microscope, repulsive van der Waals forces have been measured with higher precision than attractive van der Waals forces.2,3
1. Hamaker, H. C., Physica 4, 1058 (1937).
2. S. Lee, W. Sigmund
Repulsive van der Waals Forces for Silica and Alumina, J. Colloid Interface Science, accepted, in press 2001
3. Seung-woo Lee and Wolfgang M. Sigmund
AFM Study of Repulsive van der Waals Forces between Teflon AFTM Thin Film and Silica or Alumina, Colloids and Surfaces A, accepted 2001.
Special focus: colloid probe technique for nanosize particles
As particle size is decreased from micron size down to true nano size (<10 nm), surface forces are increasingly important.4 Additionally, continuum and mean field theories can no longer be applied. Quantum theoretical calculations are just about to reach this level. Nanoparticles at close proximity or high solids loading are expected to show a different behavior than what can be estimated from continuum and mean field theories. Using a multiwall nanotube as colloid probe is a useful approach to shed light in this size regime.5

4. W. Sigmund, N. Bell, L. Bergström, Invited Feature: Novel Powder Processing Methods for Advanced Ceramics, J. Am. Ceram. Soc., 83 (7)2000 1557-74
5. J. Cho, W. Sigmund, Direct Surface Force Measurement in Water Using a Nanosize Colloidal Probe Technique, J. Colloid Interface Science, accepted, in press 2001.
Other related publications:
L. Palmqvist, F. Lange, W. Sigmund, J. Sindel
Dispersion and consolidation of alumina using a bis-hydrophilic diblock-copolymer
J. Am. Ceram. Soc., 83 (7) 2000 1585-91.
J. Sindel, W. Sigmund, F. Aldinger
Direct measurement of interparticle forces in an aqueous barium titanate slurry
Fortschrittsberichte der DKG, 15 (1) 2000, 85-97 (in German).
Sindel, J., N.S. Bell, W.M. Sigmund
Electrolyte Effects on Nonionic Steric layers: Bis-hydrophilic Diblock-Copolymer Adsorbed on Barium Titanate
J. Am. Ceram. Soc. 82 (11) 1999 2953-57.